13. Aug. 2025
An international team led by researchers from CEITEC Masaryk University has made a breakthrough in supramolecular chemistry that could significantly improve cancer treatment. The scientists developed new molecular structures capable of more precisely delivering therapeutic agents to cancer cells, thereby increasing the effectiveness of treatment. This research has the potential to change the approach to treating cancer and other serious diseases, thanks to materials that can mimic natural processes in the body.
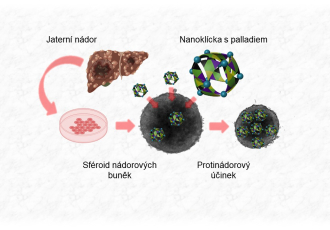
The team created so-called “supramolecular nanocages” – molecular structures designed to transport therapeutic substances directly into cancer cells. The inspiration came from natural transport processes in the human body. “We built these structures using molecules commonly found in the digestive tract – bile acids, which we slightly modified into so-called organic ligands and connected to palladium ions. They can penetrate cell membranes of liver cancer cells and release a toxic substance, thereby suppressing the viability of the malignant cells,” explains Ondřej Jurček, head of the research team at CEITEC MUNI at the time of the study.
Surprisingly, the production of these supramolecular structures is simple: the components spontaneously form a stable structure. This process, called “self-assembly”, is quick and efficient – the building blocks are dissolved, slightly heated, and within an hour the molecules arrange themselves into the nanocage structure.
“The most challenging part of this work was analysing the structure of the cage, to understand how the individual building blocks are connected. We had to combine multiple analytical methods and create models using computational chemistry until the computer model matched the product in the test tube. It’s a lengthy and demanding detective-like process,” says Subhasis Chattopadhyay, a student who participated in the research during his PhD at Masaryk University.
Another key aspect of the study was exploring how changes in conditions affect the size and structure of the cages. The team found that even minor adjustments could switch between two sizes of cages – larger and smaller (half-sized). This process is crucial for optimizing cell membrane penetration and achieving maximum effectiveness in targeting cancer cells.
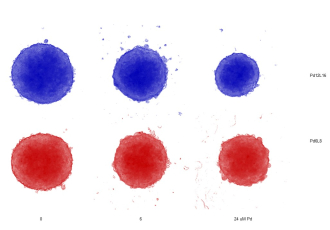
The discovery was tested in biological studies in collaboration with scientists from Masaryk University’s RECETOX research centre, yielding further surprising results: the combination of the organic ligand and palladium ion increased the uptake of toxic palladium into cancer cells by nearly 60 times, reducing their size and viability by half. Results showed that a comparable dose of palladium without the nanocage had no significant effect.
This research, conducted in collaboration with scientists from Estonia and Finland, holds great promise for society. If applied in practice, these new supramolecular structures could offer more efficient cancer therapies that are targeted and gentler on healthy tissues, potentially reducing side effects for patients and speeding recovery. In addition, these structures may inspire new therapeutic approaches in other areas of medicine.
The discovery has been published in the prestigious journal Angewandte Chemie International Edition.


 Share
Share