10. Nov. 2025
A research team from CEITEC Masaryk University (MUNI) led by Radek Marek has experimentally demonstrated for the first time – using a highly sensitive nuclear magnetic resonance (NMR) method – that even weak forces between molecules, known as halogen bonds, have a partly covalent nature, meaning that the molecules actually share electrons. In molecular sciences, this phenomenon is widely known to occur only within single molecules. The discovery offers a new perspective on how molecules interact in nature and in materials, and may help in the design and development of new drugs, catalysts, and advanced technologies.
A halogen bond occurs when a suitably polarised halogen atom (for instance, iodine or bromine) from one molecule is attracted to an atom of another molecule. Such intermolecular bonds are common in both natural and synthetic substances and play a role in processes such as molecular recognition in the body or crystal formation. These weak attractive forces are typically described as non-covalent interactions, where molecules attract each other through electric forces without sharing electrons. Covalent bonds, on the other hand, are the classical way atoms join – by sharing electron pairs to form stable molecules.
“We wanted to find out whether even very weak forces between molecules could have a touch of chemical-bond character – that is, whether electrons are shared or transferred between atoms,” says Anagha Sasikumar, the study’s first author. “Using paramagnetic NMR, we were able, for the first time, to indirectly measure electron sharing in a way that has never been observed before. This method is inherently extremely sensitive – it can detect what other techniques, such as X-ray diffraction or infrared spectroscopy, cannot see.”
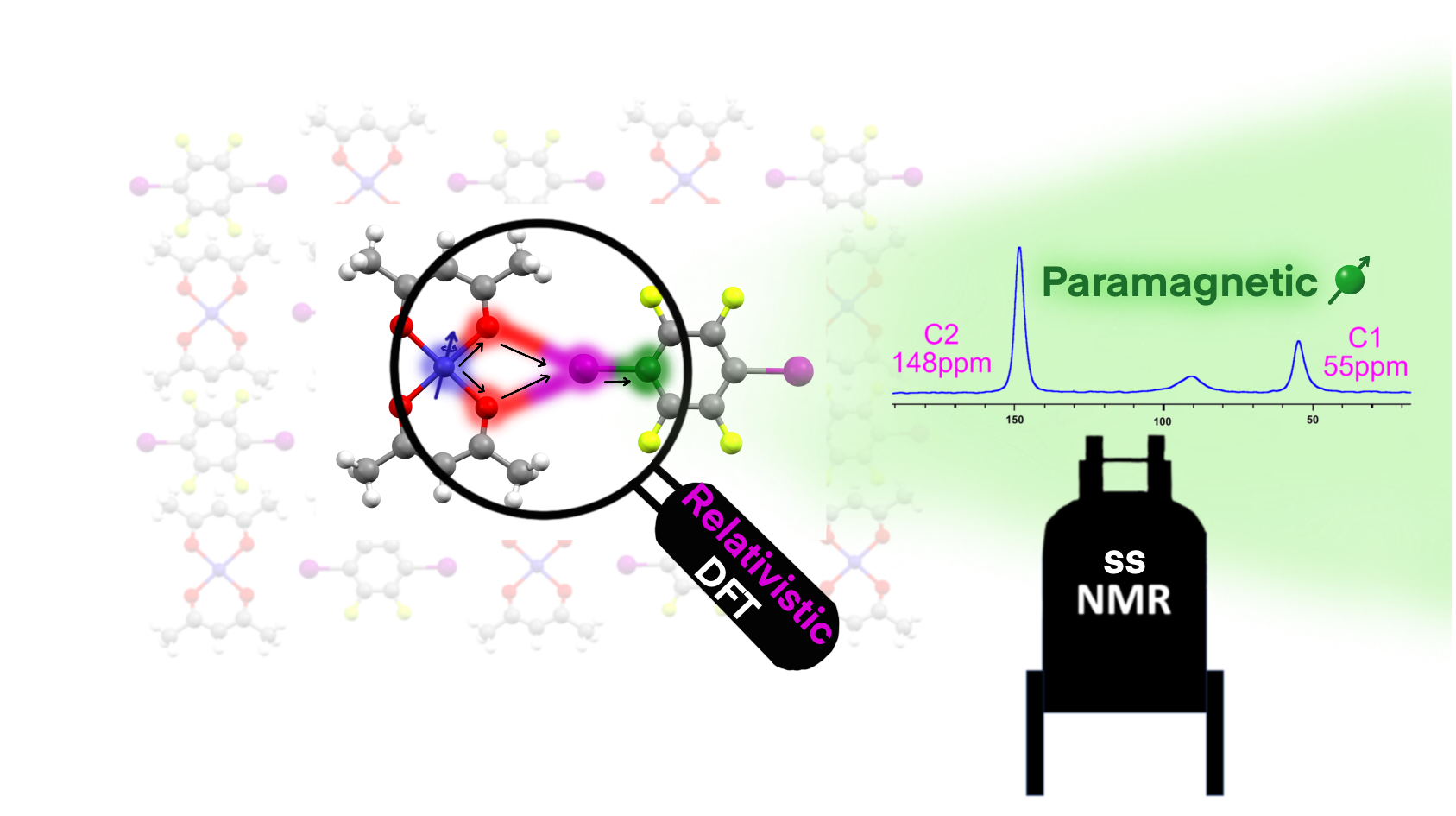
Paramagnetic NMR spectroscopy enables scientists to monitor the transfer of electron spin between atoms through a phenomenon known as the Fermi-contact interaction. This interaction allowed the team to precisely determine the extent to which molecules in a halogen bond share electrons. According to the authors, the method is not only more accurate than previous approaches but also provides a new tool for studying other types of intermolecular interactions.
The discovery confirms a previously theoretically predicted phenomenon known as supramolecular covalence. Scientists are thus blurring the boundary between “non-covalent” and “covalent” interactions – the very forces that determine how molecules recognise and bind to each other in nature and in synthetic materials. Their findings may lead to a deeper understanding of electron sharing between molecules.
“By linking the Fermi-contact NMR shift with the degree of electron sharing even in such weak supramolecular bonds, we can refine the description of the fundamental building principles of matter,” adds Radek Marek, head of the research group Structure of Biosystems and Molecular Materials at CEITEC MUNI. “In the future, we plan to apply the same method to other types of intermolecular interactions so that we can predict their behaviour and design new materials or molecules with desired properties.”
Halogen and other supramolecular bonds influence how drug molecules bind to receptors, how crystals form, or how chemical reactions proceed. A better understanding of their nature will therefore support the development of smart pharmaceuticals, more effective catalysts, and materials with tailor-made properties – from controlled drug release to molecular contacts in nanoelectronics.
The research was conducted by experts from CEITEC and the Faculty of Science, Masaryk University, in collaboration with the Institute of Macromolecular Chemistry of the Czech Academy of Sciences. The project was supported by the Czech Science Foundation (GA ČR).
The study was published in Chemical Science (Royal Society of Chemistry, 2025).


 Share
Share